Part:BBa_K4000006
HXT7p+GA+CYC1t
Profile
Name: HXT7p+GA+CYC1t
Base Pairs: 2404 bp
Origin: Saccharomyces cerevisiae, Saccharomycopsis fibuligera, synthetic
Properties: corn starch fermentation enzyme and produce sugar
Usage and Biology
Alcohol (ethanol) is an important chemical in the fields of food, medicine and bio-fuel. In particular, since the outbreak of COVID-19, the demand for alcohol for disinfection has skyrocketed [1]. Liquefaction enzyme expression in Saccharomyces cerevisiae has been reported. Bacillus amyloliquefaciens, Bacillus stearothermophilus, Bacillus Licheniformis, Bacillus subtilis Subtillis, Streptococcus Bovis, Debaryomyces Occidentali, Saccharomycopsis fibuligera and barley are common sources of α -amylase [2-3]. Because that the yeast must secrete enough amylase to support its growth at a higher initial starch concentration, and only growth can secrete enough amylase. This paradox leads to a prolonged yeast fermentation cycle, which cannot meet the requirements of industry. Based on the above studies [4-5], we considered codon optimized Glucoamylase gene (GA) derived from S. fibuligera. After elements optimization, the saccharification enzyme activity of culture supernatant of the strain obtained and the alcohol production capacity of fermented corn starch subenzyme were tested in order to construct the proper strain.
Construct design
GA (Glucoamylase) is known as the type of enzyme that can easily break down starches into glucose, which afterwards becomes usable and absorbable. we use the constitutive promoter HXT7 to regulate GA.
The profiles of every basic part are as follows:
BBa_K4000003
Name: HXT7p
Base Pairs: 587bp
Origin: Saccharomyces cerevisiae, genome
Properties: Inducible promoter regulated by glucose
Usage and Biology
HXT7 promoter is one of the high-affinity hexose transporter, it is sufficient for complementary expression of invertase to restore the growth of Saccharomyces cerevisiae in raffinose medium. HXT7 promoter could be used for heterologous protein expression in S. cerevisiae.
BBa_K40000001
Name: GA
Base Pairs: 1567bp
Origin: Saccharomycopsis fibuligera, synthetic
Properties: An enzyme that can easily break down starches into glucose
Usage and Biology
Glucoamylase is an enzyme that can be obtained from the yeast or fungi in the Aspergillus genus such as Aspergillus niger. The enzyme decomposes starch molecules in the human body into the useful energy compound of glucose. This is accomplished by removing the alpha-1 and 4-glycosidic linkages from the non-reducing end of the starch molecule. These molecules are more commonly referred to as polysaccharides and are frequently either amylase- or amylopectin-based.
Experimental approach
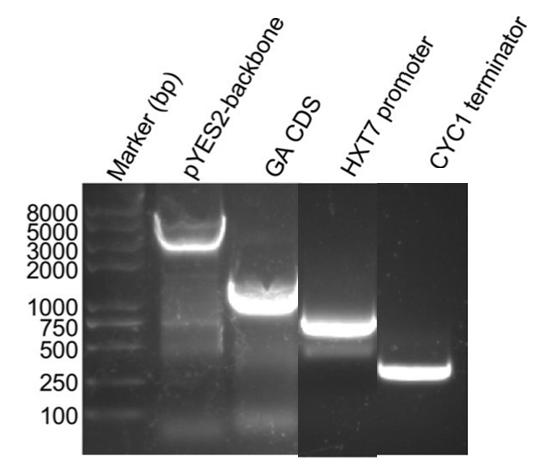
1. Fragments PCR products Electrophoresis
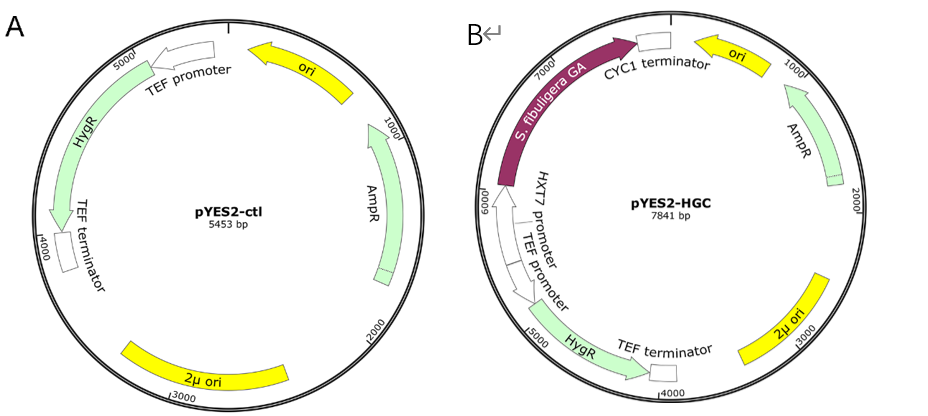
The basic parts of the plasmids such as the pYES2 backbone, GA coding sequence, HXT7 promoters, and terminators were all amplified successfully firstly. Then the multiplex GA expression cassettes were assembled using the overlap PCR method.
2. Colony PCR to identify the correct plasmids
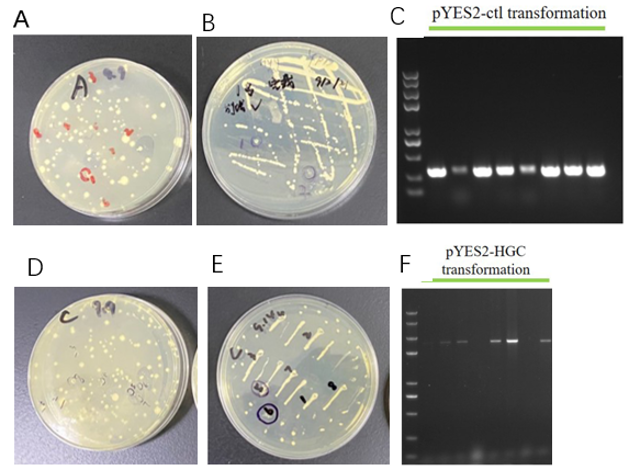
We chose 24 colonies to verify whether the plasmids were correct or not using the colony PCR, the positive rate for the plasmids pYES2-HGC were 17/24. Two or three positive colonies were sequenced to verify further.
3. GA plasmids transformation and identification
GA-expressing plasmids transformation and positive S. cerevisiae transformants selection using 50 μg/mL (Figure 5A,D) and 350 μg/mL (Figure 5B,E). The colonies which grew on the high concentration hygromycin plates were subjected to colony PCR to verify the plasmids transformation again. From Fig. 5 we can see that positive bands implied the plasmids transformation successfully.
References
1. Görgens J F, Bressler D C, van Rensburg E. Engineering Saccharomyces cerevisiae for direct conversion of raw, uncooked or granular starch to ethanol[J]. Critical reviews in biotechnology, 2015, 35(3): 369-391.
2. Van Zyl W H, Bloom M, Viktor M J. Engineering yeasts for raw starch conversion[J]. Applied microbiology and biotechnology, 2012, 95(6): 1377-1388.
3. Maury J, Kannan S, Jensen N B, et al. Glucose-dependent promoters for dynamic regulation of metabolic pathways[J]. Frontiers in bioengineering and biotechnology, 2018, 6: 63.
4. Weber E, Engler C, Gruetzner R, et al. A modular cloning system for standardized assembly of multigene constructs[J]. PloS one, 2011, 6(2): e16765.
5. Pollak B, Cerda A, Delmans M, et al. Loop assembly: a simple and open system for recursive fabrication of DNA circuits[J]. New Phytologist, 2019, 222(1): 628-640.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 718
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 679
Illegal AgeI site found at 2050 - 1000COMPATIBLE WITH RFC[1000]
| None |